Revealed: Genetic Inheritance Influences Adverse Drug Reactions
An international study led by the IBE reveals that genetic variation among human populations influences the risk of experiencing adverse drug reactions.
Using artificial intelligence, the team has discovered that populations with American and European genetic ancestry have a higher risk of experiencing toxicity from a wide range of medications.
The study suggests that better understanding the role of genetic inheritance in drug response could be key to the future of personalized medicine.
Despite humans sharing more than 99.9% of their genome, each individual is unique. Our genetic differences manifest in various ways, from eye or hair colour to disease susceptibility or how we react to medications. For instance, people with a deficiency in the G6PD gene may experience fatigue or abdominal pain, among other symptoms, when taking aspirin. In fact, adverse drug reactions (ADRs) are a leading cause of illness and mortality worldwide, ranking between the fourth and sixth most common causes of death. While genetics can affect the safety and effectiveness of drug treatments, this information is often overlooked when prescribing medications.
Now, a study led by the Institute of Evolutionary Biology (IBE), a joint centre of the Spanish National Research Council (CSIC) and the Pompeu Fabra University (UPF), has revealed key data in the journal iScience (Cell Press) about the influence of genetic inheritance on drug toxicity. Using artificial intelligence, the team analyzed 1,136 genetic variants linked to the toxicity of certain drug groups in 3,714 individuals from around the world.
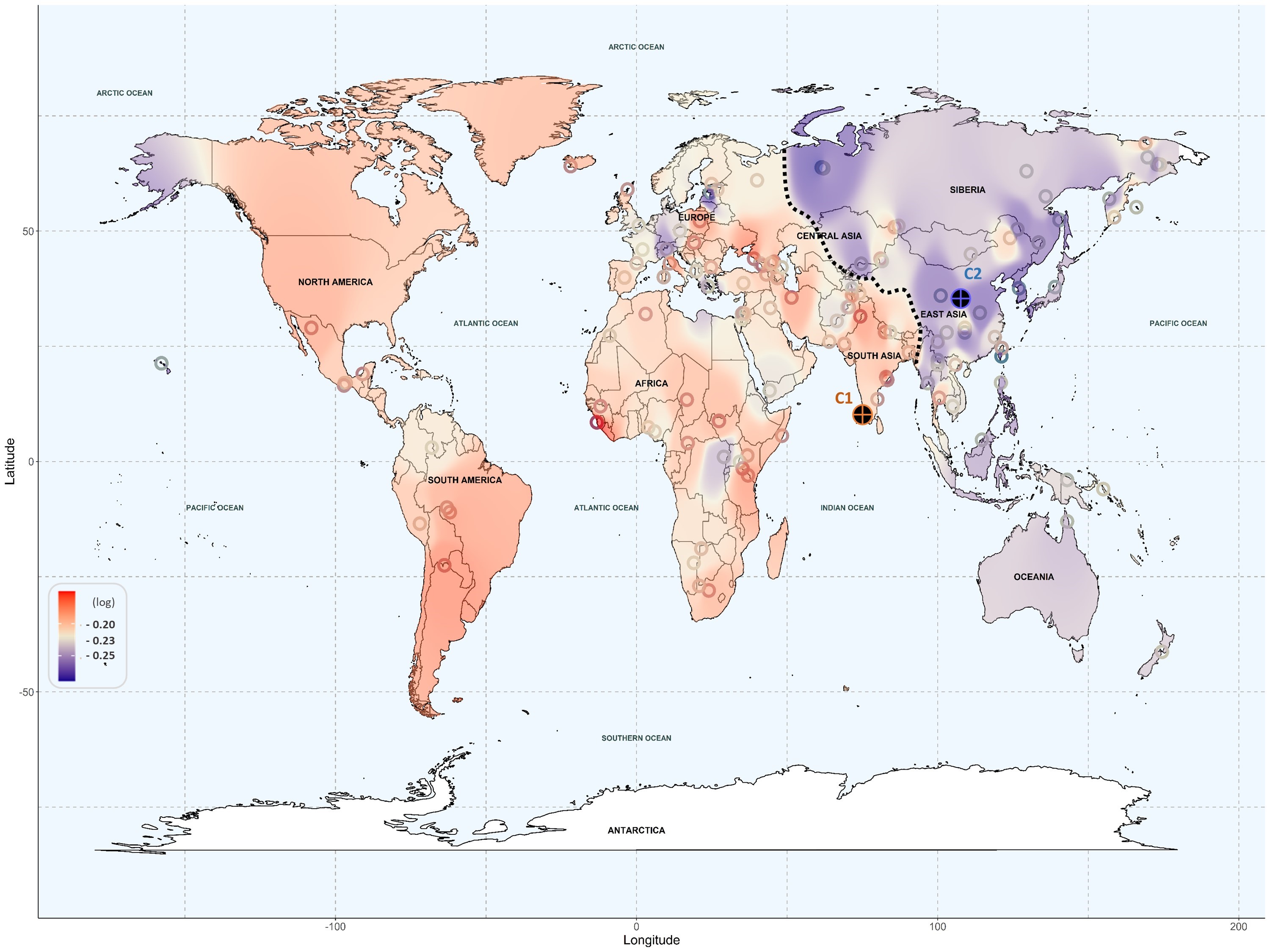
The results show that populations with American and European ancestry are at higher risk of drug toxicity, while individuals with East Asian ancestry, and to a lesser extent, Oceanic populations, show a lower risk. The study highlights the potential of genetic information from individuals and populations as a key resource in designing personalized medicine strategies.
Bringing harmful drug effects closer to resolution with new tool
To conduct their analysis, the team studied genes crucial for drug absorption and transport within the body. In particular, they used artificial intelligence tools powered by machine learning algorithms to perform a large-scale genomic analysis of 1,136 pharmacogenomic variants in 3,714 individuals from five continents.
Using text mining techniques, the research team grouped these genetic variants and linked them to the genetic ancestry of geographical regions worldwide. The team developed a new tool that reveals the key role of population genetics in drug effects. "With this large-scale study, we were able to observe, with global data, that genetic variants among individuals and populations can increase the risk of adverse drug reactions," explains Òscar Lao, the principal investigator of the Algorithms for Population Genomics Group at IBE, who led the study.
This research could have a significant impact on pharmacogenomics, a growing field in medicine that studies drug effects based on individuals' genetics, with applications in personalized medicine.
European and American Populations More Prone to Adverse Drug Reactions
The team analyzed the frequency of alleles (alternative gene forms) involved in the occurrence of adverse effects across six different drug groups. The results indicate that American and European populations are at higher risk of drug toxicity from cardiovascular and antimicrobial medications. American populations also show a higher risk for antidepressants and painkillers, while European populations are more susceptible to toxicity from immunosuppressants and anticancer drugs. On the other hand, Oceanic and Asian populations displayed lower risk across all drug groups, except for some individuals from Central Asia, who showed a higher risk for painkillers.
"Many of the drugs in the study were tested on individuals of European ancestry before being marketed. If there were a bias in this strategy, we would expect the drugs to be safer in these populations than in others, but we see the opposite, so we can rule out medical bias as the reason for these results. Everything points to the different frequencies of these genetic variants among populations having an evolutionary explanation," adds Lao.
Pharmacogenetics at the heart of personalized medicine
The study highlights pharmacogenetics as a key tool for personalized medicine: a paradigm where treatments can be tailored to each patient. "The fact that we observe differences between populations due to these variants suggests it would be valuable to include an individual's genetic ancestry when planning more personalized treatments," says Lao. In the future, simple genetic tests combined with artificial intelligence could identify patients vulnerable to severe side effects from certain medications. Ultimately, this could lead to the inclusion of each patient’s pharmacogenetic and ancestry profile as part of their medical record.
"Our results point to the potential of genetic information in human populations to personalize treatments, enhance benefits, and minimize risks and side effects," concludes Lao.
Reference article:
Karamperis et al., Genetic ancestry in population pharmacogenomics unravels distinct geographical patterns related to drug toxicity, iScience (2024), DOI:10.1016/j.isci.2024.110916
